70 Years After Hiroshima: "No government is well aware of the economic importance of biotechnology"
I was recently interviewed by Le Monde for a series on the impact of Hiroshima on science and science policy, with a particular focus on biotechnology, synthetic biology, and biosecurity. Here is the story in French. Since the translation via Google is a bit cumbersome to read, below is the English original.
Question 1
On the 16 of July 1945, after the first nuclear test at
large scale in New Mexico (called trinity) the American physicist Kenneth
Bainbridge, head of the shooting, told Robert Oppenheimer, head of the
Manhattan Project, "Now we are all sons of bitches ".
In your discipline, do you feel that the time the searchers might have the same revelation has been reached ? Will it be soon?
I think this analogy does not apply to biotechnology. It is crucially important to distinguish between weapons developed in a time of war and the pursuit of science and technology in a time of peace. Over the last thirty years, biotechnology has emerged as a globally important technology because it is useful and beneficial.
The development and maintenance of biological weapons is internationally outlawed, and has been for decades. The Trinity test, and more broadly the Manhattan Project, was a response to what the military and political leaders of the time considered an existential threat. These were actions taken in a time of world war. The scientists and engineers who developed the U.S. bombs were almost to a person ambivalent about their roles – most saw the downsides, yet were also convinced of their responsibility to fight against the Axis Powers. Developing nuclear weapons was seen as imperative for survival.
The scale of the Manhattan Project (both in personnel and as a fraction of GDP) was unprecedented, and remains so. In contrast to the exclusive governmental domain of nuclear weapons, biotechnology has been commercially developed largely with private funds. The resulting products – whether new drugs, new crop traits, or new materials – have clear beneficial value to our society.
Question 2
Do you have this feeling in other disciplines? Which ones ? Why?
No. There is nothing in our experience like the Manhattan Project and nuclear weapons. It is easy to point to the participants’ regrets, and to the long aftereffects of dropping the bomb, as a way to generate debate about, and fear of, new technologies. The latest bugaboos are artificial intelligence and genetic engineering. But neither of these technologies – even if they can be said to qualify as mature technologies – is even remotely as impactful as nuclear weapons.
Question 3
What could be the impact of a "Hiroshima" in your discipline?
In biosecurity circles, you often hear discussion of what would happen if there were “an event”. It is often not clear what that event might be, but it is presumed to be bad. The putative event could be natural or it could be artificial. Perhaps the event might kill many people as Hiroshima. (Though that would be hard, as even the most deadly organisms around today cannot wipe out populated cities in an instant.) Perhaps the event would be the intentional use of a biological weapon, and perhaps that weapon would be genetically modified in some way to enhance its capabilities. This would obviously be horrible. The impact would depend on where the weapon came from, and who used it. Was it the result of an ongoing state program? Was it a sample deployed, or stolen, from discontinued program? Or was it built and used by a terrorist group? A state can be held accountable by many means, but we are finding it challenging to hold non-state groups to account. If the organism is genetically modified, it is possible that there will be pushback against the technology. But biotechnology is producing huge benefits today, and restrictions motivated by the response to an event would reduce those benefits. It is also very possible that biotechnology will be the primary means to provide remedies to bioweapons (probably vaccines or drugs), in which case an event might wind up pushing the technology even faster.
Question 4
After 1945, physicists, including Einstein, have committed an ethical reflection on their own work. has your discipline done the same ? is it doing the same today ?
Ethical reflection has been built into biotechnology from its origins. The early participants met at Asilomar to discuss the implications of their work. Today, students involved in the International Genetically Engineered Machines (iGEM) competition are required to complete a “policy and practices” (also referred to as “ethical, legal, and social implications” (ELSI)) examination of their project. This isn’t window dressing, by any means. Everyone takes it seriously.
Question 5
Do you think it would be necessary to rase the public awarereness about the issues related to your work?
Well, I’ve been writing and speaking about this issue for 15 years, trying to raise awareness of biotechnology and where it is headed. My book, “Biology is Technology”, was specifically aimed at encouraging public discussion. But we definitely need to work harder to understand the scope and impact of biotechnology on our lives. No government measures very well the size of the biotechnology industry – either in terms of revenues or in terms of benefits – so very few people understand how economically pervasive it is already.
Question 6
What is, according to you, the degree of liberty of scientists face to political and industrial powers that will exploit the results of the scientific works?
Scientists face the same expectation of personal responsibility as every other member of the societies to which they belong. That’s pretty simple. And most scientists are motivated by ideals of truth, the pursuit of knowledge, and improving the human condition. That is one reason why most scientists publish their results for others to learn from. But it is less clear how to control scientific results after they are published. I would turn your question in another direction, and say politicians and industrialists should be responsible for how they use science, rather than putting this all on scientists. If you want to take this back to the bomb, the Manhattan Project was a massive military operation in a time of war, implemented by both government and the private sector. It relied on science, to be sure, but it was very much a political and industrial activity – you cannot divorce these two sides of the Project.
Question 7
Do you think about accurate measures [?] to prevent further Hiroshima?
I constantly think about how to prevent bad things from happening. We have to pay attention to how new technologies are developed and used. That is true of all technologies. For my part, I work domestically and internationally to make sure policy makers understand where biotechnology is headed and what it can do, and also to make sure it is not misused.
But I think the question is rather
off target. Bombing Hiroshima was a conscious decision made by an elected
leader in a time of war. It was a very specific sort of event in a very specific
context. We are not facing any sort of similar situation. If the intent of the
question is to make an analogy to intentional use of biological weapons, these
are already illegal, and nobody should be developing or storing them under any
circumstances. The current international arms control regime is the way to deal
with it. If the intent is to allude to the prevention of “bad stuff”, then this
is something that every responsible citizen should be doing anyway. All we can
do is pay attention and keep working to ensure that technologies are not used
maliciously.
Brewing Bad Biosecurity Policy
Last week brought news of a truly interesting advance in porting opioid production to yeast. This is pretty cool science, because it involves combining enzymes from several different organisms to produce a complex and valuable chemical, although no one has yet managed to integrate the whole synthetic pathway in microbes. It is also potentially pretty cool economics, because implementing opiate production in yeast should dramatically lower the price of a class of important pain medications to a point that developing countries might finally be able to afford.
Alongside the scientific article was a Commentary – with images of drug dens and home beer brewing – explicitly suggesting that high doses of morphine and other addictive narcotics would soon be brewed at home in the garage. The text advertised “Home-brew opiates” – wow, just like beer! The authors of the Commentary used this imagery to argue for immediate regulation of 1) yeast strains that can make opioids (even though no such strains exist yet), and 2) the DNA sequences that code for the opioid synthesis pathways. This is a step backward for biosecurity policy, by more than a decade, because the proposal embraces measures known to be counterproductive for security.
The wrong recipe.
I'll be very frank here – proposals like this are deep failures of the science policy enterprise. The logic that leads to “must regulate now!” is 1) methodologically flawed and 2) ignores data we have in hand about the impacts of restricting access to technology and markets. In what follows, I will deal in due course with both kinds of failures, as well as looking at the predilection to assume regulation and restriction should be the primary policy response to any perceived threat.
There are some reading this who will now jump to “Carlson is yet again saying that we should have no regulation; he wants wants everything to be available to anyone.” This is not my position, and never has been. Rather, I insist that our policies be grounded in data from the real world. And the real world data we have demonstrates that regulation and restriction often cause more harm than good. Moreover, harm is precisely the impact we should expect by restricting access to democratized biological technologies. What if even simple analyses suggests that proposed actions are likely to make things worse? What if the specific policy actions recommended in response to a threat have already been shown to exacerbate damage from the threat? That is precisely the case here. I am constantly confronted with people saying, "That's all very well and good, but what do you propose we do instead?" The answer is simple: I don't know. Maybe nothing. Maybe there isn't anything we can do. But for now, I just want us to not make things worse. In particular I want to make sure we don't screw up the emerging bioeconomy by building in perverse incentives for black markets, which would be the worst possible development for biosecurity.
Policy conversations at all levels regularly make these same mistakes, and the arguments are nearly uniform in structure. “Here is something we don't know about, or are uncertain about, and it might be bad – really, really bad – so we should most certainly prepare policy options to prevent the hypothetical worst!” Exclamation points are usually just implied throughout, but they are there nonetheless. The policy options almost always involve regulation and restriction of a technology or process that can be construed as threatening, usually with little or no consideration of what that threatening thing might plausibly grow into, nor of how similar regulatory efforts have fared historically.
This brings me to the set up. Several news pieces (e.g., the NYT, Buzzfeed) succinctly pointed out that the “home-brew” language was completely overblown and inflammatory, and that the Commentary largely ignored both the complicated rationale for producing opioids in yeast and the complicated benefits of doing so. The Economist managed to avoid getting caught up in discussing the Commentary, remaining mostly focussed on the science, while in the last paragraph touching on the larger market issues and potential future impacts of “home brew opium” to pull the economic rug out from under heroin cartels. (Maybe so. It's an interesting hypothesis, but I won't have much to say about it here.) Over at Biosecu.re, Piers Millet – formerly of the Biological Weapons Convention Implementation Support Unit – calmly responded to the Commentary by observing that, for policy inspiration, the authors look backward rather than forward, and that the science itself demonstrates the world we are entering requires developing completely new policy tools to deal with new technical and economic realities.
Stanford's Christina Smolke, who knows a thing or two about opioid production in yeast, observed in multiple news outlets that getting yeast to produce anything industrially at high yields is finicky to get going and then hard to maintain as a production process. It's relatively easy to produce trace amounts of lots of interesting things in microbes (ask any iGEM team); it is very hard and very expensive to scale up to produce interesting amounts of interesting things in microbes (ask any iGEM team). Note that we are swimming in data about how hard this is to do, which is an important part of this story. In addition to the many academic examples of challenges in scaling up production, the last ten years are littered with startups that failed at scale up. The next ten years, alas, will see many more.
Even with an engineered microbial strain in hand, it can be extraordinarily hard to make a microbe jump through the metabolic and fermentation hoops to produce interesting/useful quantities of a compound. And then transferring that process elsewhere is very frequently its own expensive and difficult effort. It is not true that you can just mail a strain and a recipe from one place to another and automatically get the same result. However, it is true that all this will get easier over time, and many people are working on reproducible process control for biological production.
That future looks amazing. I've written many times about how the future of the economy looks like beer and cows – in other words, that our economy will inevitably be based on distributed biological manufacturing. But that is the future: i.e., not the present. Nor is it imminent. I truly wish it were imminent, but it is not. Whole industries exist to solve these problems, and much more money and effort will be spent before we get there. The economic drivers are huge. Some of the investments made by Bioeconomy Capital are, in fact, aimed at eventually facilitating distributed biological manufacturing. But, if nothing else, these investments have taught me just how much effort is required to reach that goal. If anybody out there has a credible plan to build the Cowborg or to microbrew chemicals and pharmaceuticals as suggested by the Commentary, I will be your first investor. (I said “credible”! Don't bother me otherwise.) But I think any sort of credible plan is years away. For the time being, the only thing we can expect to brew like beer is beer.
FBI Supervisory Special Agent Ed You makes great use of the “brewing bad” and “baking bad” memes, mentioned in the Commentary, in talking to students and professionals alike about the future of drug production. But this is in the context of taking personal responsibility for your own science and for speaking up when you see something dangerous. I've never heard Ed say anything about increasing surveillance and enforcement efforts as the way forward. In fact, in the Times piece, Ed specifically says, “We’ve learned that the top-down approach doesn’t work.” I can't say exactly why Ed chose that turn of phrase, but I can speculate that it is based 1) on his own experience as a professional bench molecular biologist, 2) the catastrophically bad impacts of the FBI's earlier arrests and prosecutions of scientists and artists for doing things that were legal, and 3) the official change in policy from the White House and National Security Council away from suppression and toward embracing and encouraging garage biology. The standing order at the FBI is now engagement. In fact, Ed You's arrival on the scene was coincident with any number of positive policy changes in DC, and I am happy to give him all the credit I can. Moreover, I completely agree with Ed and the Commentary authors that we should be discussing early on the implications of new technologies, an approach I have been advocating for 15 years. But I completely disagree with the authors that the current or future state of the technology serves as an indicator of the need to prepare some sort of regulatory response. We tried regulating fermentation once before; that didn't work out so well [1].
Badly baked regulatory policy.
So now we're caught up to about the middle of the Commentary. At this point, the story is like other such policy stories. “Assume hypothetical thing is inevitable: discuss and prepare regulation.” And like other such stories, here is where it runs off the rails with a non sequitur common in policy work. Even if the assumption of the thing's inevitability is correct (which is almost always debatable), the next step should be to assess the impact of the thing. Is it good, or is it bad? (By a particular definition of good and bad, of course, but never mind that for now.) Usually, this question is actually skipped and the thing is just assumed to be bad and in need of a policy remedy, but the assumption of badness, breaking or otherwise, isn't fatal for the analysis.
Let's say it looks bad – bad, bad, bad – and the goal of your policy is to try to either head it off or fix it. First you have to have some metric to judge how bad it is. How many people are addicted, or how many people die, or how is the crime rate affected? Just how bad is it breaking? Next – and this is the part the vast majority of policy exercises miss – you have to try to understand what happens in the absence of a policy change. What is the cost of doing nothing, of taking no remediating action? Call this the null hypothesis. Maybe there is even a benefit to doing nothing. But only now, after evaluating the null hypothesis, are you in a position to propose remedies, because only now you have a metric to compare costs and benefits. If you leap directly to “the impacts of doing nothing are terrible, so we must do something, anything, because otherwise we are doing nothing”, then you have already lost. To be sure, policy makers and politicians feel that their job is to do something, to take action, and that if they are doing nothing then they aren't doing their jobs. That is just a recipe for bad policy. Without the null hypothesis, your policy development is a waste of time and, potentially, could make matters worse. This happens time and time again. Prohibition, for example, was exactly this sort of failure, and cost much more than it benefited, which is why it was considered a failure [2].
We keep making the same mistake. We have plenty of data and reporting, courtesy of the DEA, that the ongoing crackdown on methamphetamine production has created bigger and blacker markets, as well as mayhem and violence in Mexico, all without much impact on domestic drug use. Here is the DEA Statistics & Facts page – have a look and then make up your own mind.
I started writing about the potential negative impacts of restricting access to biological technologies in 2003 (PDF), including the likely emergence of black markets in the event of overregulation. I looked around for any data I could find on the impacts of regulating democratized technologies. In particular, I happened upon the DEA's first reporting of the impacts of the then newly instituted crackdown on domestic methamphetamine production and distribution. Even in 2003, the DEA was already observing that it had created bigger, blacker markets – that are by definition harder to surveil and disrupt – without impacting meth use. The same story has played out similarly in cocaine production and distribution, and more recently in the markets for “bath salts”, aka “legal highs”.
That is, we have multiple, clear demonstrations that, rather than improving the world, restricting access to distributed production can instead cause harm. But, really, when has this ever worked? And why do people think going down the same path in the future will lead anywhere else? I am still looking for data – any data at all – that supports the assertion that regulating biological technologies will have any different result. If you have such data, bring it. Let's see it. In that absence of that data, policy proposals that lead with regulation and restriction are doomed to repeat the failures of the past. It has always seemed to me like a terrible idea to transfer such policies over to biosecurity. Yet that is exactly what the Commentary proposes.
Brewing black markets.
The fundamental problem with the approach advocated in the Commentary is that security policies, unlike beer brewing, do not work equally well across all technical and economic scales. What works in one context will not work in another. Nuclear weapons can be secured by guns, gates, and guards because they are expensive to build and the raw materials are hard to come by, so heavy touch regulation works just fine. There are some industries – as it happens, beer brewing – where only light touch regulation works. In the U.S., we tried heavy touch regulation in the form of Prohibition, and it failed miserably, creating many more problems than it solved. There are other industries, for example DNA and gene synthesis, in which even light touch regulations are a bad idea. Indeed, light touch regulation of has already created the problem it was supposed to prevent, namely the existence of DNA synthesis providers that 1) intentionally do not screen their orders and 2) ship to countries and customers that are on unofficial black lists.
For those who don't know this story: In early 2013, the International Council for the Life Sciences (ICLS) convened a meeting in Hong Kong to discuss "Codes of Conduct" for the DNA synthesis industry, namely screening orders and paying attention to who is doing the ordering. According to various codes and guidelines promulgated by industry associations and the NIH, DNA synthesis providers are supposed to reject orders that are similar to sequences that code for pathogens, or genes from pathogens, and it is suggested that they do not ship DNA to certain countries or customers (the unofficial black list). Here is a PDF of the meeting report; be sure to read through Appendix A.
The report is fairly anodyne in describing what emerged in discussions. But people who attended have since described in public the Chinese DNA synthesis market as follows. There are 3 tiers of DNA providers. The first tier is populated with companies that comply with the various guidelines and codes promulgated internationally because this tier serves international markets. There is a second tier that appears to similarly comply, because while they serve primarily the large internal market these companies have aspirations of also serving the international market. There is a third tier that exists specifically to serve orders from customers seeking ways around the guidelines and codes. (One company in this tier was described to me as a "DNA shanty", with the employees living over the lab.) Thus the relatively light touch guidelines (which are not laws) have directly incentivized exactly the behavior they were supposed to prevent. This is not a black market, per se, and cannot be accurate described as illegal, so let's call it a "grey market".
I should say here that this is entirely consistent with my understanding of biotech in China. In 2010, I attended a warm up meeting for the last round of BWC negotiations. After that meeting, I chatted with one of the Chinese representatives present, hoping to gain a little bit of insight into the size of the Chinese bioeconomy and the state of the industry. My query was met with frank acknowledgment that the Chinese government isn't able to keep track of the industry, does't know how many companies are active, or how many employees they have, or what they are up to, and so doesn't hold out much hope of controlling the industry. I covered this a bit in my 2012 Biodefense Net Assessment report for DHS. (If anyone has any new insight into the Chinese biotech industry, I am all ears.) Not that the U.S. or Europe is any better in this regard, as our mechanisms for tracking the biotech industry are completely dysfunctional, too. There could very well be DNA synthesis providers operating elsewhere that don't comply with the recommended codes of conduct: we have no real means of broadly surveying for this behavior. There are no physical means either to track it remotely or to control it.
I am a little bit sensitive about the apparent emergence of the DNA synthesis grey market, because I warned for years in print and in person that DNA screening would create exactly this outcome. I was condescendingly told on many occasions that it was foolish to imagine a black market for DNA. And then we have to do something, right? But it was never very complicated to think this through. DNA is cheap, and getting cheaper. You need this cheap DNA as code to build more complicated, more valuable things. Ergo, restrictions on DNA synthesis will incentivize people to seek, and to provide, DNA outside any control mechanism. The logic is pretty straightforward, and denying it is simply willful self-deception. Regulation of DNA synthesis will never work. In the vernacular of the day: because economics. To make it even simpler: because humans.
So the idea that people are still suggesting proscription of certain DNA sequences is a viable route to security just rankles. And it is demonstrably counterproductive. The restrictions incentivize the bad behavior they are supposed to prevent, probably much earlier than might have happened otherwise. The take home message here is that not all industries are the same, because not all technologies are the same, and that our policy approaches should take into account these differences rather than papering over them. In particular, restricting access to information in our modern economy is a losing game.
Where do we go from here?
We are still at the beginning of biotech. This is the most important time to get it right. This is the most important time not to screw up and make things worse. And it is important that we are at the beginning, because things are not yet screwed up.
Conversely, we are well down the road in developing and deploying drug policies, with much damage done. To be sure, despite the accumulated and ongoing costs, you have to acknowledge that it is not at all clear that suddenly legalizing drugs such as meth or cocaine would be a positive step. I am not in any way making that argument. But it is abundantly clear that drug enforcement activities have created the world we live in today. Was there an alternative? If the DEA had been able to do cost/benefit analysis of the impacts of its actions – that is, predict the emergence of DTOs and their role in production, trafficking, and violence – would the policy response 15 years ago have been any different? If Nixon had more thoughtfully considered even what was known 50 years about about the impacts of proscription, would he have launched the war on drugs? That is a hard question, because drug policy is clearly driven more by stories and personal politics than by facts. I am inclined to think the present drug policy mess was inevitable. Even with the DEA's self-diagnosed role in creating and sustaining DTOs, the national conversation is still largely dominated by “the war on drugs”. And thus the first reaction to the prospect of microbial narcotics production is to employ strategies and tactics that have already failed elsewhere. I would hate to think we are in for a war on microbes, because that is doomed to failure.
But we haven't yet made all those mistakes with biological technologies. I continue to hope that, if nothing else, we will avoid making things worse by rejecting policies we already know won't work.
Notes:
[1] Pause here to note that even this early in the set up, the Commentary conflates via words and images the use of yeast in home brew narcotics with centralized brewing of narcotics by cartels. These are two quite different, and are perhaps mutually exclusive, technoeconomic futures. Drug cartels very clearly have the resources to develop technology. Depending on whether you listen to the U.S. Navy or the U.S. Coast Guard, either 30% or 80% of the cocaine delivered to the U.S. is transported at some point in semisubmersible cargo vessels or in fully submersible cargo submarines. These 'smugglerines', if you will, are the result of specific technology development efforts directly incentivized by governmental interdiction efforts. Similarly, if cartels decide that developing biological technologies suits their business needs, they are likely to do so. And cartels certainly have incentives to develop opioid-producing yeast, because fermentation usually lowers the cost of goods between 50% and 90% compared to production in plants. Again, cartels have the resources, and they aren't stupid. If cartels do develop these yeast strains, for competitive reasons they certainly won't want anyone else to have them. Home brew narcotics would further undermine their monopoly.
[2] Prohibition was obviously the result of a complex socio-political situation, just as was its repeal. If you want a light touch look at the interaction of the teetotaler movement, the suffragette movement, and the utility of Prohibition in continued repression of freed slaves after the Civil War, check out Ken Burns's “Prohibition” on Netflix. But after all that, it was still a dismal failure that created more problems than it solved. Oh, and Prohibition didn't accomplish its intended aims. Anheuser-Busch thrived during those years. Its best selling products at the time were yeast and kettles (see William Knoedleseder's Bitter Brew)...
Announcing Bioeconomy Capital
I am pleased to announce the launch of Bioeconomy Capital. Our investments so far are:
- Riffyn, which is building software that provides experimental process design and analytics software to improve reproducibility and tech transfer in life science and materials R&D;
- Synthace, which is increasing the reliability, quality, and scale of biological science;
- RoosterBio, which is is creating exponential advances in stem cell manufacturing to provide raw materials for cell-based therapies, biofabrication, and cellular ink for 3D BioPrinting.
2014 U.S. Brewery Count Chart
As usual, note that the x-axis is decadal until 2010, and that I have tacked individual years on to the right side of the chart.
Here is the PDF for Microbrewing the Bioeconomy (2011).
Biosecurity is Everyone's Business (Part 2)
(Here is Part 1.)
Part 2. From natural security to neural security
Humans are fragile. For most of history we have lived with the expectation that we will lose the use of organs, and some of us limbs, as we age or suffer injury. But that is now changing. Prostheses are becoming more lifelike and more useful, and replacement organs have been used to save lives and restore function. But how robust are the replacement parts? The imminent prospect of technological restoration of human organs and limbs lost to injury or disease is cause to think carefully about increasing both our biological capabilities and our technological fragilities.
Technology fails us for many reasons. A particular object or application may be poorly designed or poorly constructed. Constituent materials may be faulty, or maintenance may be shoddy. Failure can result from inherent security flaws, which can be exploited directly by those with sufficient technical knowledge and skill. Failure can also be driven by clever and conniving exploits of the overall system that focus on its weakest link, almost always the human user, by inducing them to make a mistake or divulge critical information. Our centuries of experience and documentation of such failures should inform our thinking about the security of emerging technologies, particularly as we begin to fuse biology with electronic systems. The growing scope of biotechnology will therefore require constant reassessment of what vulnerabilities we are introducing through that expansion. Examining the course of other technologies provides some insight into the future of biology.
We carry powerful computers in our pockets, use the internet to gather information and access our finances, and travel the world in aircraft that are often piloted and landed by computers. We are told we can trust this technology with our financial information, our identities and social networks, and, ultimately, our lives. At the same time, technology is constantly shown to be vulnerable and fragile at a non-trivial rate -- resulting in identity theft, financial loss, and sometimes personal injury and death. We embrace technology despite well-understood risks; automobiles, electricity, fossil fuels, automation, and bicycles all kill people every day in predictable numbers. Yet we continue to use technology, integrating it further into multiple arenas in our lives, because we decide that the benefits outweigh risks.
Healthcare is one arena in which risks are multiplying. The IT security community has for some years been aware of network vulnerabilities in medical devices such as pacemakers and implantable defibrillators. The ongoing integration of networked medical devices in health care settings, an integration that is constantly introducing both new capabilities and new vulnerabilities, is now the focus of extensive efforts to improve security. The impending introduction of networked, semi-autonomous prostheses raises obvious similar concerns. Wi-fi enabled pacemakers and implantable defibrillators are just the start, as soon we will see bionic arms, legs, and eyes with network connections that allow performance monitoring and tuning.
Eventually, prostheses will not simply restore "human normal" capabilities, they will also augment human performance. I learned recently that DARPA explicitly chose to limit the strength of its robotic arm, but that can't last: science fiction, super robotic strength is coming. What happens when hackers get ahold of this technology? How will people begin to modify themselves and their robotic appendages? And, of course, the flip side of having enhanced physical capabilities is having enhanced vulnerabilities. By definition, tuning can improve or degrade performance, and this raises an important security question: who holds the password for your shiny new arm? Did someone remember to overwrite the factory default password? Is the new password susceptible to a dictionary attack? The future brings even more concerns. Control connections to a prosthesis are bi-directional and, as the technology improves, ever better neural interfaces will eventually jack these prostheses directly into the brain. "Tickling" a robotic limb could take on a whole new meaning, providing a means to connect various kinds of external signals to the brain in new ways.
Beyond limbs, we must also consider neural connections that serve to open entirely novel senses. It is not a great leap to envision a wide range of ensuing digital-to-neural input/output devices. These technologies are evolving at a rapid rate, and through them we are on the cusp of opening up human brains to connections with a wide range of electromechanical hardware capabilities and, indeed, all the information on the internet.
Just this week saw publication of a cochlear implant that delivers a gene therapy to auditory neurons, promoting the formation of electrical connections with the implant and thereby dramatically improving the hearing response of test animals. We are used to the idea of digital music files being converted by speakers into sound waves, which enter the brain through the ear. But the cochlear implant is basically an ethernet connection wired to your auditory nerve, which in principal means any signal can be piped into your brain. How long can it be before we see experiments with a cochlear (or other) implant that enables direct conversion of arbitrary digital information into neural signals? At that point, "hearing" might extend into every information format. So, again we must ask, who holds the password to your brain implant
Hacking the Bionic Man
As this technology is deployed in the population it is clear that there can be no final and fixed security solution. Most phone and computer users are now all too aware that new hardware, firmware, and operating systems always introduce new kinds of risks and threats. The same will be true of prostheses. The constant rat race to chase down security holes in new products upgrades will soon extend directly into human brains. As more people are exposed to medical device vulnerabilities, security awareness and improvement must become an integrated part of medical practice. This discussion can be easily extended to potential vulnerabilities that will arise from the inevitable integration into human bodies of not just electromechanical devices, but of ever more sophisticated biological technologies. The exploration of prosthesis security, loosely defined, gives some indication of the scope of the challenge ahead.
The class of things we call prostheses will soon expand beyond electromechanical devices to encompass biological objects such as 3D printed tissues and lab-grown organs. As these cell-based therapies begin to enter human clinical trials, we must assess the security of both the therapies themselves and the means used to create and administer them. If replacement organs and tissues are generated from cells derived from donors, what vulnerabilities do the donors have? How are those donor vulnerabilities passed along to the recipients? Yes, you have an immune system that does wonders most of the time. But are your natural systems up to the task of handling the biosecurity of augmented organs?
What does security even mean in this context? In addition to standard patient work-ups, should we begin to fully sequence the genomes of donor tissues, first to identify potential known health issues, and then to build a database that can be re-queried as new genetic links to disease are discovered? Are there security holes in the 3D printers and other devices used to manipulate cells and tissues? What are the long term security implications of deploying novel therapeutic tissues in large numbers of military and civilian personnel? What are the long-term security implications of using both donor and patient tissue as seeds of induced pluripotent stem cells, or of differentiating any stem cell line for use in therapies? Do we fully understand the complement of microbes and genomes that may be present in donor samples, or lying dormant in donor genomes, or that may be introduced via laboratory procedures and instruments used to process cells for use as therapies? What is the genetic security of a modified cell line or induced pluripotent stem cell? If there is a genetic modification embedded in your replacement heart tissue, where did the new DNA come from, and are you sure you know everything that it encodes? As with information technologies, we should expect that these new biological technologies will sometimes arrive with accidental vulnerabilities; they may also come with intentionally introduced back doors. The economic motivation to create new protheses, as well as to exploit vulnerabilities, will soon introduce market competition as a factor in biosecurity.
Competition often drives perverse strategic decisions when it comes to security. Firms rush to sell hardware and software that are said to be secure, only to discover that constant updates are required to patch security holes. We are surrounded by products in endless beta. Worse yet, manufacturers have been known to sit on security holes in the naive hope that no one else will notice. Vendors sometimes appear no more literate about the security of hardware and software than are their customers. What will the world look like when eletromechanical and biological prostheses are similarly in constant states of upgrade? Who will you trust to build/print/grow a prosthesis? Are you going to place your faith in the FDA to police all these risks? (Really?) If you decide to instead place your faith in the market, how will you judge the trustworthiness of firms that sell aftermarket security solutions for your bionic leg or replacement liver?
The complexity of the task at hand is nearly overwhelming. Understanding the coming fusion of technologies will require competency in software, hardware, wetware, and security -- where are those skill sets being developed in a compatible, integrated manner? This just leads to more questions: Are there particular countries that will have a competitive advantage in this area? Are there particular countries that will be hotbeds of prosthesis malware creation and distribution?
The conception of security, whether of individuals or nation states, is going to change dramatically as we become ever more economically dependent upon the market for biological technologies. Given the spreading capability to participate and innovate in technology development, which inevitably amplifies the number and effect of vulnerabilities of all kinds, I suspect we need to re-envision at a very high level how security works.
[Coming soon: Part 3.]
Biosecurity is Everyone's Business (Part 1)
Part 1. The ecosystem is the enterprise
We live in a society increasingly reliant upon the fruits of nature. We consume those fruits directly, and we cultivate them as feedstocks for fuel, industrial materials, and the threads on our backs. As a measure of our dependence, revenues in the bioeconomy are rising rapidly, demonstrating a demand for biological products that is growing much faster than the global economy as a whole.
This demand represents an enormous market pull on technology development, commercialization, and, ultimately, natural resources that serve as feedstocks for biological production. Consequently, we must assess carefully the health and longevity of those resources. Unfortunately, it is becoming ever clearer that the natural systems serving to supply our demand are under severe stress. We have been assaulting nature for centuries, with the heaviest blows delivered most recently. Nature, in the most encompassing sense of the word, has been astonishingly resilient in the face of this assault. But the accumulated damage has cracked multiple holes in ecosystems around the globe. There are very clear economic costs to this damage -- costs that compound over time -- and the cumulative damage now poses a threat to the availability of the water, farmland, and organisms we rely on to feed ourselves and our economy.
I would like to clarify that I am not predicting collapse, nor that we will run out of resources; rather, I expect new technologies to continue increasing productivity and improving the human condition. Successfully developing and deploying those technologies will, obviously, further increase our economic dependency on nature. As part of that growing dependency, businesses that participate in the bioeconomy must understand and ensure the security of feedstocks, transportation links, and end use, often at a global scale. Consequently, it behooves us to thoroughly evaluate any vulnerabilities we are building into the system so that we can begin to prepare for inevitable contingencies.
Revisiting the definition of biosecurity: from national security to natural security, and beyond
Last year John Mecklin at Bulletin of the Atomic Scientists asked me to consider the security implications of the emerging conversation (or, perhaps, collision) between synthetic biology and conservation biology. This conversation started at a meeting last April at the University of Cambridge, and is summarized in a recent article in Oryx. What I came up with for BAS was an essay that cast very broadly the need to understand threats to all of the natural systems we depend on. Quantifying the economic benefit of those systems, and the risk inherent in our dependence upon them, led me directly to the concept of natural security.
Here I want to take a stab at expanding the conversation further. Rapidly rising revenues in the bioeconomy, and the rapidly expanding scope of application, must critically inform an evolving definition of biosecurity. In other words, because economic demand is driving technology proliferation, we must continually refine our understanding of what it is that we must secure and from where threats may arise.
Biosecurity has typically been interpreted as the physical security of individuals, institutions, and the food supply in the context of threats such as toxins and pathogens. These will, of course, continue to be important concerns: new influenza strains constantly emerge to cause human and animal health concerns; the (re?)emergent PEDS virus has killed an astonishing 10% of U.S. pigs this year alone; within the last few weeks there has been an alarming uptick in the number of human cases and deaths caused by MERS. Beyond these natural threats are pathogens created by state and non-state organizations, sometimes in the name of science and preparation for outbreaks, while sometimes escaping containment to cause harm. Yet, however important these events are, they are but pieces of a biosecurity puzzle that is becoming ever more complex.
Due to the large and growing contribution of the bioeconomy, no longer are governments concerned merely with the proverbial white powder produced in a state-sponsored lab, or even in a 'cave' in Afghanistan. Because economic security is now generally included in the definition of national security, the security of crops, drug production facilities, and industrial biotech will constitute an ever more important concern. Moreover, in the U.S., as made clear by the National Strategy for Countering Biological Threats(PDF), the government has established that encouraging the development and use of biological technologies in unconventional environments (i.e., "garages and basements") is central to national security. Consequently, the concept of biosecurity must comprise the entire value chain from academics and garage innovators, through production and use, to, more traditionally, the health of crops, farmanimals, and humans. We must endeavor to understand, and to buttress, fragility at every link in this chain.
Beyond the security of specific links in the bioeconomy value chain we must examine the explicit and implicit connections between them, because through our behavior we connect them. We transport organisms around the world; we actively breed plants, animals, and microbes; we create new objects with flaws; we emit waste into the world. It's really not that complicated. However, we often choose to ignore these connections because acknowledging them would require us to respect them, and consequently to behave differently. But that change in behavior must be the future of biosecurity.
From an enterprise perspective, as we rely ever more heavily on biology in our economy, so must we comprehensively define 'biosecurity' to adequately encompass relevant systems. Vulnerabilities in those systems may be introduced intentionally or accidentally. An accidental vulnerability may lie undiscovered for years, as in the case of the recently disclosed Heartbleed hole in the OpenSSL internet security protocol, until it is identified, when it becomes a threat. The risk, even in open source software, is that the vulnerability may be identified by organizations which then exploit it before it becomes widely known. This is reported to be true of the NSA's understanding and exploitation of Heartbleed at least two years in advance of its recent public announcement. Our biosecurity challenge is to carefully, and constantly, assess how the world is changing and address shortcomings as we find them. It will be a transition every bit as painful as the one we are now experiencing for hardware and software security
(Here is Part 2.)
Using programmable inks to build with biology: mashing up 3D printing and biotech
Scientists and engineers around the globe dream of employing biology to create new objects. The goal might be building replacement organs, electronic circuits, living houses, or cowborgs and carborgs (my favorites) that are composed of both standard electromechanical components and novel biological components. Whatever the dream, and however outlandish, we are getting closer every day.
Looking a bit further down the road, I would expect organs and tissues that have never before existed. For example, we might be able to manufacture hybrid internal organs for the cowborg that process rough biomass into renewable fuels and chemicals. Both the manufacturing process and the cowborg itself might utilize novel genetic pathways generated in DARPA's Living Foundries program. The first time I came across ideas like the cowborg was in David Brin's short story "Piecework". I've pondered this version of distributed biological manufacturing for years, pursuing the idea into microbrewing, and then to the cowborg, the economics of which I am now exploring with Steve Aldrich from bio-era.
Yet as attractive and powerful as biology is as a means for manufacturing, I am not sure it is powerful enough. Other ways that humans build things, and that we build things that build things, are likely to be part of our toolbox well into the future. Corrosion-resistant plumbing and pumps, for example, constitute very useful kit for moving around difficult fluids, and I wouldn't expect teflon to be produced biologically anytime soon. Photolithography, electrodeposition, and robotics, now emerging in the form of 3D printing, enable precise control over the position of matter, though frequently using materials and processes inimical to biology. Humans are really good at electrical and mechanical engineering, and we should build on that expertise with biological components.
Let's start with the now hypothetical cowborg. The mechanical part of a cowborg could be robotic, and could look like Big Dog, or perhaps simply a standard GPS-guided harvester, which comes standard with air conditioning and a DVD player to keep the back-up human navigation system awake. This platform would be supplemented by biological components, initially tanks of microbes, that turn raw feedstocks into complex materials and energy. Eventually, those tanks might be replaced by digestive organs and udders that produce gasoline instead of milk, where the artificial udders are enabled by advances in genetics, microbiology, and bioprinting. Realizing this vision could make biological technologies part of literally anything under the sun. In a simple but effective application along these lines, the ESA is already using "burnt bone charcoal" as a protective coating on a new solar satellite.
But there is one persistent problem with this vision: unless it is dead and processed, as in the case of the charcoal spacecraft coating, biology tends not to stay where you put it. Sometimes this will not matter, such as with many replacement transplant organs that are obviously supposed to be malleable, or with similar tissues made for drug testing. (See the recent Economist article, "Printing a bit of me", this CBS piece on Alexander Seifalian's work at University College London, and this week's remarkable news out of Anthony Atala's lab.) Otherwise, cells are usually squishy, and they tend to move around, which complicates their use in fabricating small structures that require precise positioning. So how do you use biology to build structures at the micro-scale? More specifically, how do you get biology to build the structures you want, as opposed to the structures biology usually builds?
We are getting better at directing organisms to make certain compounds via synthetic biology, and our temporal control of those processes is improving. We are inspired by the beautiful fabrication mechanisms that evolution has produced. Yet we still struggle to harness biology to build stuff. Will biological manufacturing ever be as useful as standard machining is, or as flexible as 3D printing appears it will be? I think the answer is that we will use biology where it makes sense, and we will use other methods where they make sense, and that in combination we will get the best of both worlds. What will it mean when we can program complex matter in space and time using a fusion of electromechanical control (machining and printing) biochemical control (chemistry and genetics)? There are several recent developments that point the way and demonstrate hybrid approaches that employ the 3D printing of biological inks that subsequently display growth and differentiation.
Above is a slide I used at the recent SynBERC retreat in Berkeley. On the upper left, Organovo is now shipping lab-produced liver tissue for drug testing. This tissue is not yet ready for use in transplants, but it does display all the structural and biochemical complexity of adult livers. A bit further along in development are tracheas from Harvard Biosciences, which are grown from patient stem cells on 3D-printed scaffolds (Claudia Castillo was the first recipient of a transplant like this in 2007, though her cells were grown on a cadaver windpipe first stripped of the donor's cells). And then we have the paper on the right, which really caught my eye. In that publication, viruses on a 3D woven substrate were used to reprogram human cells that were subsequently cultured on that substrate. The green cells above, which may not look like much, are the result of combining 3D fabrication of non-living materials with a biological ink (the virus), which in combination serve to physically and genetically program the differentiation and growth of mammalian cells, in this case into cartilage. That's pretty damn cool.
Dreams of building with biology
Years ago, during the 2003 "DARPA/ISAT Synthetic Biology Study", we spent considerable time discussing whether biology could be used to rationally build structures like integrated circuits. The idea isn't new: is there a way to get cells to build structures at the micro- or nano-scale that could help replace photolithography and other 2D patterning techniques used to build chips? How can humans make use of cells -- lovely, self-reproducing factories -- to construct objects at the nanometer scale of molecules like proteins, DNA, and microtubules?
Cells, of course, have dimensions in the micron range, and commercial photolithography was, even in 2003, operating at about the 25 nanometer range (now at about 15 nm). The challenge is to program cells to lay down structures much smaller than they are. Biology clearly knows how to do this already. Cells constantly manufacture and use complex molecules and assemblies that range from 1 to 100 nm. Many cells move or communicate using extensions ("processes") that are only 10-20 nanometers in width but tens microns in length. Alternatively, we might directly use synthetic DNA to construct a self-assembling scaffold at the nano-scale and then build devices on that scaffold using DNA-binding proteins. DNA origami has come a long way in the last decade and can be used to build structures that span nanometers to microns, and templating circuit elements on DNA is old news. We may even soon have batteries built on scaffolds formed by modified, self-assembling viruses. But putting all this together in a biological package that enables nanometer-scale control of fabrication across tens of centimeters, and doing it as well as lithography, and as reproducibly as lithography, has thus far proved difficult.
Conversely, starting at the macro scale, machining and 3D printing work pretty well from meters down to hundreds of microns. Below that length scale we can employ photolithography and other microfabrication methods, which can be used to produce high volumes of inexpensive objects in parallel, but which also tend to have quite high cost barriers. Transistors are so cheap that they are basically free on a per unit basis, while a new chip fab now costs Intel about $10 billion.
My experiences working on different aspects of these problems suggest to me that, eventually, we will learn to exploit the strengths of each of the relevant technologies, just as we learn to deal with their weaknesses; through the combination of these technologies we will build objects and systems that we can only dream of today.
Squishy construction
Staring through a microscope at fly brains for hours on end provides useful insights into the difference between anatomy and physiology, between construction and function. In my case, those hours were spent learning to find a particular neuron (known as H1) that is the output of the blowfly motion measurement and computation system. The absolute location of H1 varies from fly to fly, but eventually I learned to find H1 relative to other anatomical landmarks and to place my electrode within recording range (a few tens of microns) on the first or second try. It's been long known that the topological architecture (the connectivity, or wiring diagram) of fly brains is identical between individuals of a given species, even as the physical architecture (the locations of neurons) varies greatly. This is the difference between physiology and anatomy.
The electrical and computational output of H1 is extremely consistent between individuals, which is what makes flies such great experimental systems for neurobiology. This is, of course, because evolution has optimized the way these brains work -- their computational performance -- without the constraint that all the bits and pieces must be in exactly the same place in every brain. Fly brains are constructed of squishy matter, but the computational architecture is quite robust. Over the last twenty years, humans have learned to grow various kinds of neurons in dishes, and to coax them into connecting in interesting ways, but it is usually very hard to get those cells to position themselves physically exactly where you want them, with the sort of precision we regularly achieve with other forms of matter.
Crystalline construction
The first semiconductor processing instrument I laid hands on in 1995 was a stepper. This critical bit of kit projects UV light through a mask, which contains the image of a structure or circuit, onto the surface of a photoresist-covered silicon wafer. The UV light alters the chemical structure of the photoresist, which after further processing eventually enables the underlying silicon to be chemically etched in a pattern identical to the mask. Metal or other chemicals can be similarly patterned. After each exposure, the stepper automatically shifts the wafer over, thereby creating an array of structures or circuits on each wager. This process enables many copies of a chip to be packed onto a single silicon wafer and processed in parallel. The instruments on which I learned to process silicon could handle ~10 cm diameter wafers. Now the standard is about 30 cm, because putting more chips on a wafer reduces marginal processing costs. But it isn't cheap to assemble the infrastructure to make all this work. The particular stepper I used (this very instrument, as a matter of fact), which had been donated to the Nanofabrication Facility at Cornell and which was ancient by the time I got to it, contained a quartz lens I was told cost about $1 million all by itself. The kit used in a modern chip fab is far more expensive, and the chemical processing used to fabricate chips is quite inimical to cells. Post-processing, silicon chips can be treated in ways that encourages cells to grow on them and even to form electrical connections, but the overhead to get to that point is quite high.
Arbitrary construction
The advent of 3D printers enables the reasonably precise positioning of materials just about anywhere. Depending on how much you want to spend, you can print with different inks: plastics, composites, metals, and even cells. This lets you put stuff where you want it. The press is constantly full of interesting new examples of 3D printing, including clothes, bone replacements, objects d'art, gun components, and parts for airplanes. As promising as all this is, the utility of printing is still limited by the step size (the smallest increment of the position of the print head) and the spot size (the smallest amount of stuff the print head can spit out) of the printer itself. Moreover, printed parts are usually static: once you print them, they just sit there. But these limitations are already being overcome by using more complex inks.
Hybrid construction
If the ink used in the printer has the capacity to change after it gets printed, then you have introduced a temporal dimension into your process: now you have 4D printing. Typically, 4D printing refers to objects whose shape or mechanical properties can be dynamically controlled after construction, as with these 3D objects that fold up after being printed as 2D objects. But beyond this, if you combine squishy, crystalline, and arbitrary construction, you get a set of hybrid construction techniques that allows programming matter from the nanoscale to the macroscale in both time and space.
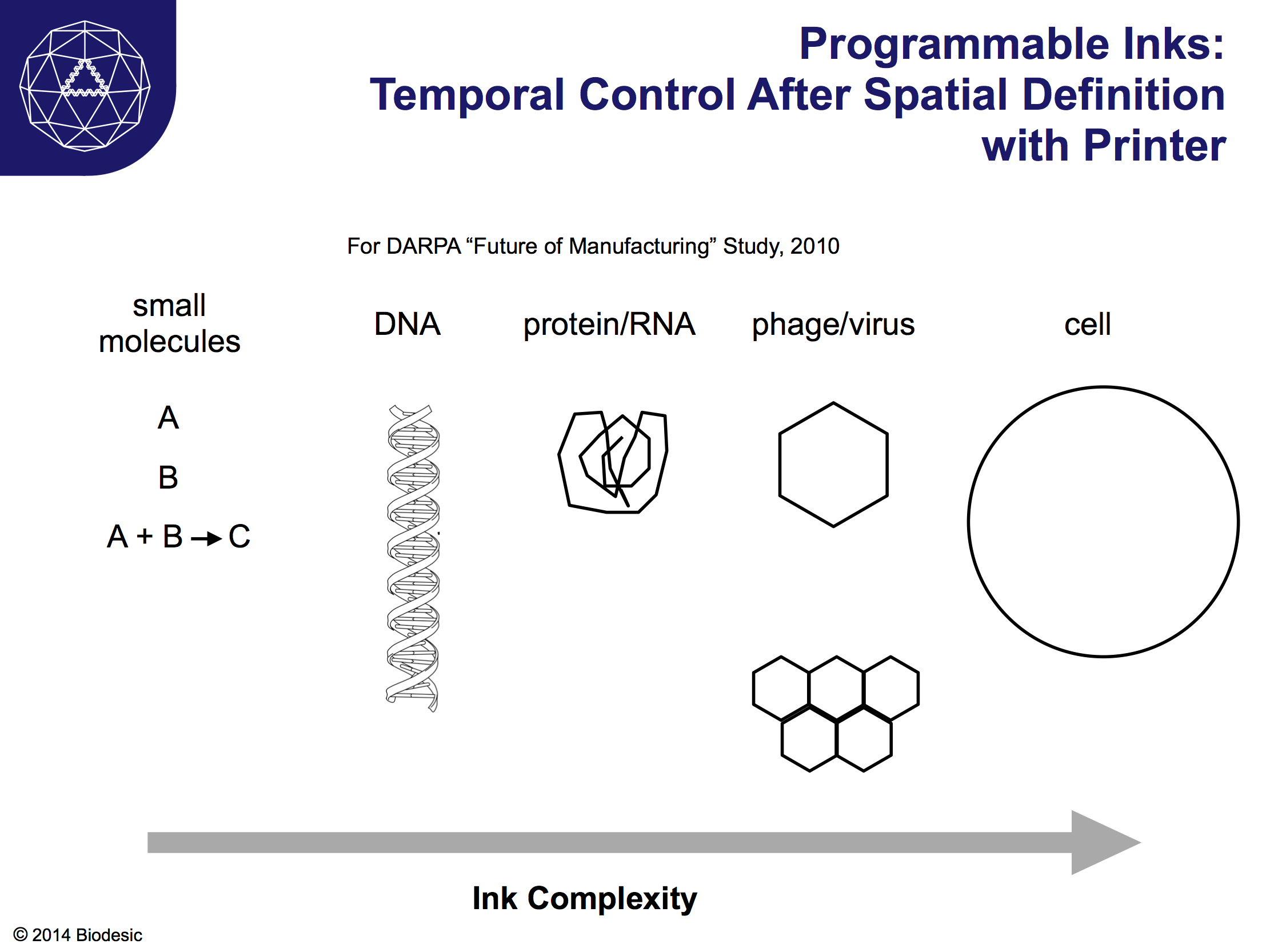
Above is a slide from a 2010 DARPA study on the Future of Manufacturing, from a talk in which I tried to articulate the utility of mashing up 3D printing and biotech. We have already seen the first 3D printed organs, as described earlier. Constructed using inks that contain cells, even the initial examples are physiologically similar to natural organs. Beyond tracheas, printed or lab-growth organs aren't yet ready for general use as transplants, but they are already being used to screen drugs and other chemicals for their utility and toxicity. Inks could also consist of: small molecules (i.e. chemicals) that react with each other or the environment after printing; DNA and proteins that serve structural, functional (say, electronic), or even genetic roles after printing; viruses that form structures or are that are intended to interact biologically with later layers; cells that interact with each other or follow some developmental program defined genetically or by the substrate, as demonstrated in principle by the cartilage paper above.
The ability to program the three-dimensional growth and development of complex structures will have transformative impacts throughout our manufacturing processes, and therefore throughout our economy. The obvious immediate applications include patient-specific organs and materials such as leather, bone, chitin, or even keratin (think vat-grown ivory), that are used in contexts very different than we are used to today.
It is hard to predict where this is going, of course, but any function we now understand for molecules or cells can be included in programmable inks. Simple 2-part chemical reactions will eventually be common in inks, eventually transitioning to more complex inks containing multiple reactants, including enzymes and substrates. Eventually, programmable printer inks will employ the full complement of genes and biochemistry present in viruses, bacteria, and eukaryotic cells. Beyond existing genetics and biochemistry, new enzymes and genetic pathways will provide materials we have never before laid hands on. Within DARPA's Living Foundries program is the 1000 Molecules program, which recently awarded contracts to use biology to generate "chemical building blocks for accessing radical new materials that are impossible to create with traditional petroleum-based feedstocks".
Think about that for a moment: it turns out that of the entire theoretical space of compounds we can imagine, synthetic chemistry can only provide access to a relatively small sample. Biology, on the other hand, in particular novel systems of (potentially novel) enzymes, can be programmed to synthesize a much wider range of compounds. We are just learning how to design and construct these pathways; the world is going to look very different in ten years' time. Consequently, as these technologies come to fruition, we will learn to use new materials to build objects that may be printed at one length scale, say centimeters, and that grow and develop at length scales ranging from nanometers to hundreds of meters.
Just as hybrid construction that combines the features of printers and inks will enable manufacturing on widely ranging length scales, so will it give us access to a wide range of time scales. A 3D printer presently runs on fairly understandable human time scales of seconds to hours. For the time being, we are still learning how to control print heads and robotic arms that position materials, so they move fairly slowly. Over time, the print head will inevitably be able to move on time scales at least as short as milliseconds. Complex inks will then extend the reach of the fabrication process into the nanoseconds on the short end, and into the centuries on the long end.
I will be the first to admit that I haven't the slightest idea what artifacts made in this way will do or look like. Perhaps we will build/grow trees the size of redwoods that produce fruit containing libations rivaling the best wine and beer. Perhaps we will build/grow animals that languidly swim at the surface of the ocean, extracting raw materials from seawater and photosynthesizing compounds that don't even exist today but that critical to the future economy.
These examples will certainly prove hopelessly naive. Some problems will turn out to be harder than they appear today, and other problems will turn out to be much easier than they appear today. But the constraints of the past, including the oft-uttered phrase "biology doesn't work that way", do not apply. The future of engineering is not about understanding biology as we find it today, but rather about programming biology as we will build it tomorrow.
What I can say is that we are now making substantive progress in learning to manipulate matter, and indeed to program matter. Science fiction has covered this ground many times, sometimes well, sometimes poorly. But now we are doing it in the real world, and sketches like those on the slides above provide something of a map to figure out where we might be headed and what our technical capabilities will be like many years hence. The details are certainly difficult to discern, but if you step back a bit, and let your eyes defocus, the overall trajectory becomes clear.
This is a path that John von Neumann and Norbert Wiener set out on many decades ago. Physics and mathematics taught us what the rough possibilities should be. Chemistry and materials science have demonstrated many detailed examples of specific arrangements of atoms that behave physically in specific ways. Control theory has taught us both how organisms behave over time and how to build robots that behave in similar ways. Now we are learning to program biology at the molecular level. The space of the possible, of the achievable, is expanding on a daily basis. It is going to be an interesting ride.
The most important paragraph of The Gene Factory
The most important paragraph of Michael Specter's story about BGI:
Time for New DNA Synthesis and Sequencing Cost Curves (corrected pub date)
[Given the mix-up in the publication date of 2015, I have now deleted the original post. I have appended the comments from the original post to the bottom of this post.]
It's time once again to see how quickly the world of biological technologies is changing. The story is mixed, in part because it is getting harder to find useful data, and in part because it is getting harder to generate appropriate metrics.
Sequencing and synthesis productivity
I'll start with the productivity plot, as this one isn't new. For a discussion of the substantial performance increase in sequencing compared to Moore's Law, as well as the difficulty of finding this data, please see
. If nothing else, keep two features of the plot in mind: 1) the consistency of the pace of Moore's Law and 2) the inconsistency and pace of sequencing productivity. Illumina appears to be the primary driver, and beneficiary, of improvements in productivity at the moment, especially if you are looking at share prices. It looks like the recently announced NextSeq and Hiseq instruments will provide substantially higher productivities (hand waving, I would say the next datum will come in another order of magnitude higher), but I think I need a bit more data before officially putting another point on the plot. Based on
Eric Check Hayden's coverage at Nature
, it seems that the new instruments should also provide substantial price improvements, which I get into below.
As for synthesis productivity, there have been no new commercially available instruments released for many years. sDNA providers are clearly pushing productivity gains in house, but no one outside those companies has access to productivity data.

DNA sequencing and synthesis prices
The most important thing to notice about the plots below is that prices have stopped falling precipitously. If you hear or read anyone asserting that costs are falling exponentially, you can politely refer them to the data (modulo the putative performance of the new Illumina instruments). We might again see exponential price decreases, but that will depend on a combination of technical innovation, demand, and competition, and I refer the reader to
. Note that prices not falling isn't necessarily bad and doesn't mean the industry is somehow stagnant. Instead, it means that revenues in these sectors are probably not falling, which will certainly be welcomed by the companies involved. As I described a couple of weeks ago, and
in November,
revenues in biotech continue to climb steeply
.
The second important thing to notice about these plots is that I have changed the name of the metric from "cost" to "price". Previously, I had decided that this distinction amounted to no real difference for my purposes. Now, however, the world has changed, and cost and price are very different concepts for anyone thinking about the future of DNA. Previously, there was at times an order of magnitude change in cost from year to year, and keeping track of the difference between cost and price didn't matter. In a period when change is much slower, that difference becomes much more important. Moreover, as the industry becomes larger, more established, and generally more important for the economy, we should all take more care in distinguishing between concepts like cost
to whom
and price
for whom
.
In the plot that follows, the price is for finished, not raw, sequencing.

And here is a plot only of oligo and gene-length DNA:

What does all this mean? Illumina's instruments are now responsible for such a high percentage of sequencing output that the company is effectively setting prices for the entire industry. Illumina is being pushed by competition to increase performance, but this does not necessarily translate into lower prices. It doesn't behoove Illumina to drop prices at this point, and we won't see any substantial decrease until a serious competitor shows up and starts threatening Illumina's market share. The absence of real competition is the primary reason sequencing prices have flattened out over the last couple of data points.
I pulled the final datum on the sequencing curve from the NIH; the title on the NIH curve is "cost", but as it includes indirect academic costs I am going to fudge and call it "price". I notice that the NIH is now
publishing two sequencing cost curves
, and I'll bet that the important differences between them are too subtle for most viewers. One curve shows cost per megabase of raw sequence - that is, data straight off the instruments - and the other curve shows cost per
finished
human genome (assuming ~30X coverage of 3x10^9 bases). The cost per base of that finished sequencing is a couple orders of magnitude higher than the cost of the raw data. On the
, Illumina has boldly put a point on the cost per human genome curve at $1000. But I have left it off the above plot for the time being; the performance and cost claimed by Illumina are just for human genomes rather than for arbitrary
de novo
sequencing. Mick Watson dug into this, and his sources inside Illumina claim that
this limitation is in the software
, rather than the hardware or the wetware, in which case a relatively simple upgrade could dramatically expand the utility of the instrument. Or perhaps the "de novo sequencing level" automatically unlocks after you spend $20 million in reagents. (Mick also has some strong opinions about the role of competition in pushing the development of these instruments, which I got into
.)
Synthesis prices have slowed for entirely different reasons. Again, I have covered this ground in many other
, so I won't belabor it here.
Note that the oligo prices above are for column-based synthesis, and that oligos synthesized on arrays are much less expensive. However, array synthesis comes with the usual caveat that the quality is generally lower, unless you are getting your DNA from Agilent, which probably means you are getting your
.
Note also that the distinction between the price of oligos and the price of double-stranded sDNA is becoming less useful. Whether you are ordering from Life/Thermo or from your local academic facility, the cost of producing oligos is now, in most cases, independent of their length. That's because the cost of capital (including rent, insurance, labor, etc) is now more significant than the cost of goods. Consequently, the price reflects the cost of capital rather than the cost of goods. Moreover, the cost of the columns, reagents, and shipping tubes is certainly more than the cost of the atoms in the sDNA you are ostensibly paying for. Once you get into longer oligos (substantially larger than 50-mers) this relationship breaks down and the sDNA is more expensive. But, at this point in time, most people aren't going to use longer oligos to assemble genes unless they have a tricky job that doesn't work using short oligos.
Looking forward, I suspect oligos aren't going to get much cheaper unless someone sorts out how to either 1) replace the requisite human labor and thereby reduce the cost of capital, or 2) finally replace the phosphoramidite chemistry that the industry relies upon. I know people have been talking about new synthesis chemistries for many years, but I have not seen anything close to market.
Even the cost of double-stranded sDNA depends less strongly on length than it used to. For example, IDT's
come at prices that are constant across quite substantial ranges in length. Moreover, part of the decrease in price for these products is embedded in the fact that you are buying smaller chunks of DNA that you then must assemble and integrate into your organism of choice. The longer gBlocks come in as low as ~$0.15/base, but you have to spend time and labor in house in order to do anything with them. Finally, so far as I know, we are still waiting for Gen9 and Cambrian Genomics to ship DNA at the prices they have suggested are possible.
How much should we care about the price of sDNA?
I recently had a chat with someone who has purchased and assembled an absolutely enormous amount of sDNA over the last decade. He suggested that if prices fell by another order of magnitude, he could switch completely to outsourced assembly. This is an interesting claim, and potentially an interesting "tipping point". However, what this person really needs is not just sDNA, but sDNA integrated in a particular way into a particular genome operating in a particular host. And, of course, the integration and testing of the new genome in the host organism is where most of the cost is. Given the wide variety of emerging applications, and the growing array of hosts/chassis, it isn't clear that any given technology or firm will be able to provide arbitrary synthetic sequences incorporated into arbitrary hosts.
Consequently, as I have described
, I suspect that we aren't going to see a huge uptake in orders for sDNA until cheaper genes and circuits are coupled to reductions in cost for the rest of the build, test, and measurement cycle. Despite all the talk recently about organism fabs and outsourced testing, I suggest that what will really make a difference is providing every lab and innovator with adequate tools and infrastructure to do their own complete test and measurement. We should look to progress in pushing all facets of engineering capacity for biological systems, not just on reducing the cost of reading old instructions and writing new ones.